Photochemical
Photography (Film)
A
widespread technology for recording the information at
the focal plane of a lens is something that everyone is
extremely familiar with - film. Although it may be a common,
everyday item, its roots stem directly from various, typically
complicated chemical processes. However, in order to understand
how film "records photons", it is important
to understand certain aspects of the underlying architecture
of film.
Each
roll of film that would be typically purchased on the
market today has dozens of extremely thin photochemical
layers stacked on top of a layer of a base material such
as celluloid or polyester (The Photographic Process 1,
How Photographic Film Works 4). The base is essentially
the component that all the other layers and chemicals
are adhered to. This base element only exists because
otherwise the film would be so fragile that it would tear
apart inside the camera due to mechanical strain. It has
no effect on the overall photochemical processes. The
second component of film is a rather surprising one -
gelatin. This component is used to bind all of the individual
photochemical layers together and, ultimately, to bind
these layers to the base material (How Photographic Film
Works 4). An illustration of these layers can be seen
below in figure one.
Figure
One (Illustration of the typical layering scheme present
in photographic film)
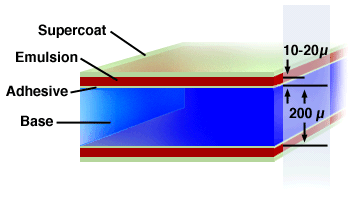
(Image courtesy of The
Photographic Process (Dr. Gambhir))
In
the figure above, the supercoat is typically a protective
coating that keeps the film from being damaged during
development of the film. The emulsion layer consists of
the few dozen layers that are key to the photochemical
processes. The adhesive is the gelatin, and the base is
the celluloid.
The
most interesting part of the film, of course, is what
lies within the layers contained in the emulsion. Within
a few of these one to two dozen layers are small silver-halide
crystal grains, which are typically created from bromide,
chloride, and iodide (the number of layers typically varies
between different manufacturers/types of film). These
crystals are the workhorses of the photochemical world,
and are directly analogous to the pixels on CCD chips.
Like CCD's, some of the crystals need to be sensitive
to red, green, and blue. However, Silver-halide crystals
are only naturally sensitive to blue light, so special
organic molecules called "spectral sensitizes"
are added to the surfaces of the crystals. These organic
molecules increase the silver halide crystal sensitivity
to red, green, and blue light, depending on which organic
molecule is added. These molecules must then release an
electron to the silver halide crystal when it is struck
with red, green, or blue light (How Photographic Film
Works 4).
In
order to understand just how this electron is freed and
where they are freed from, consider a typical silver bromide
crystal lattice as seen below in figure two.
Figure
Two (Crystal lattice of a typical silver bromide crystal)
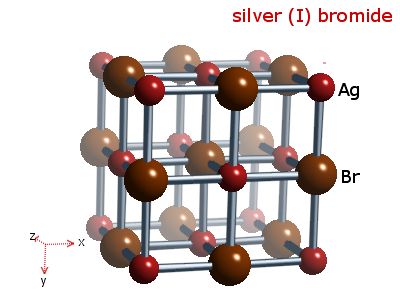
(Image courtesy of webelements.com
from Influences
of Silver Halide Crystal..)
This
lattice contains ions of bromine and silver (Br- and Ag+).
When incoming light hits this crystal lattice, the extra
electron contained on the bromide ion is released. This
free electron then jumps from the now bromine atom to
the positively charged silver ion. As a consequence of
this electron coming into contact and binding to the silver
ion, the silver ion is transformed into metallic silver
(Ag). This creates a small region of silver metal (CS39J
Session Seven 1). When this is occurring all over the
film at different regions of the focal plane and at different
intensities, a latent image is produced out of silver
atoms (essentially creating an extremely faint
black and white image). A diagram of this electron becoming
dislodged can be seen below in figure three.
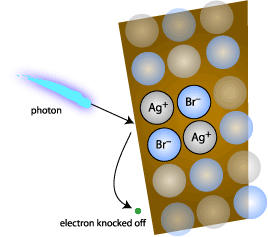
Figure
Three (Illustration of a photon hitting a bromide ion
and freeing an electron)
(Image
courtesy of
CS39J
Session Seven)
Once
this silver atom has been left behind, development of
the film enhances this latent image and produces the colors
from the different layers of film. Color is produced from
development from the fact that one layer contains silver
halide crystals that have been organically sensitized
to red, another to blue, and another to green. Development
of the film takes into account the concentrations of silver
atoms at each location everywhere on each layer. A region
with a higher concentration of silver atoms in the red
plane as opposed to blue and green will turn up as a red
color when developed. As an example of the concept, imagine
three of these crystals stacked on top of one another
within the different planes of the film (let's say the
top one is sensitive to red, the middle to green, and
the bottom to blue). If a photon of red light (i.e. if
a photon that contains the energy level of that found
in red light, related to E=hf) hits the stack of three
crystals, the crystal most sensitive to red will be activated
and will thus create a silver atom in that location of
the plane. When this region is then developed, the red
will be present at that location (i.e. a pixel location).
As
stated previously, there are other varieties of crystal
structures found in common films. Another such crystal
lattice (silver iodide) can be seen below in figure four.
Figure
Four (Crystal lattice of a typical silver iodide crystal)
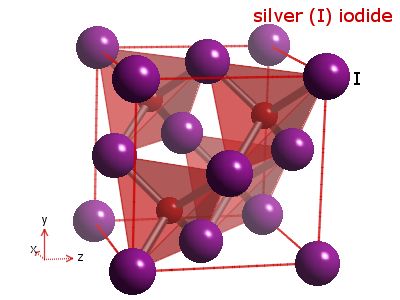
(Image courtesy of webelements.com
from Influences
of Silver Halide Crystal..)
There
are some interesting properties that are naturally inherent
to the lattices seen in figure four and figure two. For
instance, these lattices can be made larger or smaller,
depending on the will of the film manufacturer. Larger
crystals will allow for the film to be exposed more quickly,
but at the cost of decreased resolution (i.e. pixel density,
as a larger crystal will be absorbing more surface area
on the focal plane and hence the pixel/surface area ratio
will be smaller) while smaller crystals expose more slowly,
but offer higher resolution (higher pixel/surface area
ratio) (The Photographic Process 1). However,
these exposure times are relative, as small crystals with
certain chemicals fused into their lattice will expose
faster than a larger crystal without such a chemical fused
into it.

